Density of Benzoic Acid G Ml
If any need welcome ask us freely. A student dissolves 17 g of benzoic acid C7 H602 and 350 mL of a solvent with the density of 116 g mL.
13 gcm³ Solubility in water g100ml at 20C.

. Specific Gravity 12659gmL 20C. Calculate the molarity and molality of the students solution. So mass of 425.
When filling a buret always never sometimesx2 pour the sample into the buret using a. A student dissolves 12. Science Chemistry QA Library Calculate the molality of a solution that contains 979 g of benzoic acid C6H5COOH in 4190 mL of ethanol C2H5OH.
Flammability class Combustible IIIB estimatedFlammability class Molecular formula C7-H6-O2Molecular formula Molecular weight 12213 gmolMolecular weight Oxidizing properties Not oxidizing. So to obtained the heat released from the combustion of benzoic acid we just calculate the heat required to raise the temperature of the water. Calculate the molality of a solution that contains 565 mathrmg of benzoic acid mathrmC_6 mathrmH_5 mathrmCOOH in 350 mathrmmL of ethanol mathrmC_2 mathrmH_5 mathrmOH The density of ethanol is 0789 mathrmg.
So the mass of 165 mL of chloroform would be. Volume 200 L 2000 mL. It is classified as a strong acid and can attack the skin.
The density of chloroform is 14832 gmL at 25C I stole this number from a handbook. We know density is the ratio of mass to volume. Besides its most common use in refining metals hydrochloric acid is an important chemical used in the production of organic compounds such as.
Measuring out this volume is rather simple. Hydrochloric acid is an inorganic acid with the chemical formula HCl. Molar mass of benzoic acid 12212 gmol.
G of benzoic acid of benzoic acid 011 mol of benzoic acid. Of a solvent with a density of. 249390 C at 760 mmHg Vapour Pressure.
ML of solvent Molarity is defined as number of moles of solute dissolved in 1000 mL of solution. The solution in water is a weak acid. Mass 1 2000 2000 g.
We supply Benzoic acid fit for feedfood uses in flexible particle sizes. Tetrahedron Letters 37 p. 263890 C at 760 mmHg Vapour Pressure.
C 7 H 6 O 2 C 6 H 5 COOH Molecular mass. 5 of benzoic acid CHO in 300 ml. Molecular Weight Of Benzoic Acid.
The student notices that the volume of the solvent does not change when the benzoic acid dissolves in it. Chemsrc provides benzoic acidCAS65-85-0 MSDS density melting point boiling point structure formula molecular weight etc. Chemistry questions and answers.
Of a solvent with a density of 10 gml. Benzoic Acid - cas 65-85-0 synthesis structure density melting point boiling point. What is the density of benzoic acid.
Density of methanol is 0792 gmL Molar mass of CH3OH is 3204 gmol Molar mass of C6H5COOH is 12212 gmol. Round both of your answers to 2 significant digits. Density 1 gmL.
211 ºC 20 mmHg Density. When rinsing a buret always never sometimesx1 fill the buret completely with the sample and then pour out. Benzoic acid is.
Here solute is benzoic acid and total volume of solution is 425. The Journal of Organic Chemistry 27 p. A student dissolves 14g of benzoic acid C7H6O2 in 425mL of a solvent with a density of 092 gmL.
029 Vapour pressure Pa. Round both of your answers to 2 significant digits. Molarity - 0 molality - 0.
502016 BENZOIC ACID SAFETY DATA SHEET Version 04 Revision Date. Intensive and extensive are properties are characteristics of elements and compounds such as color density odor conductivity etc. What is the mole fraction Χ of CH3OH methanol in a solution of 900 mL of CH3OH and 679 g of C6H5COOH benzoic acid.
07-06-2021 Page 6 of 10 Explosive properties Not explosive. Please contact us for product TSD typical CoA and MSDS. It is a colorless liquid which has a distinctive pungent smell.
Physical and Chemical Properties Molecular formula C6H5COOH. Vapor Density air1 421. The student notices that the volume of the solvent does not change when the benzoic acid dissolves in it.
MSDS 9300 Benzoic Acid Scholar Chemistry Section 9. The student notices that the volume of the solvent does not change when the benzoic acid dissolves in it. To calculate the mass of water Density mass volume Mass Density volume.
All we need do is then calculate how much benzoic acid to weigh out. Calculate the molarity and molality of the students solution. The student notices that the volume of the solvent does not change when the benzoic acid dissolves in it.
The density of ethanol is 0789 gmL. 0006 mmHg at 25C Enthalpy of Vaporization. The density of ethanol is 0789 gmL.
We also supply coated Benzoic acid specialized in swine feed additive.

Ouai Travel Size Texturizing Hair Spray Ulta Beauty Travel Size Products Ouai Dry Shampoo

Lys Beauty No Limits Matte Bronzer Courage 0 23 Oz 6 5 G Matte Bronzer Skin Moisturizer Bronzer
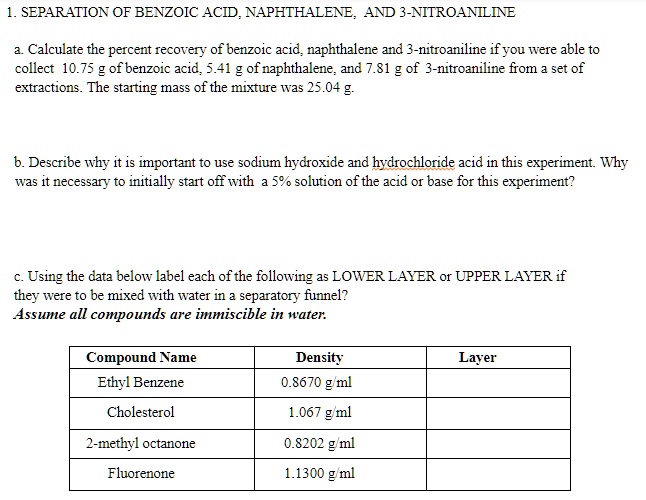
Solved Separation Of Benzoic Acid Naphthalene And 3 Nitroaniline Calculate The Percent Recovery Of Benzoic Acid Naphthalene And Nitroaniline If You Were Able To Collect 10 75 G Ofbenzoic Acid 5 41 Of Naphthalene And 81
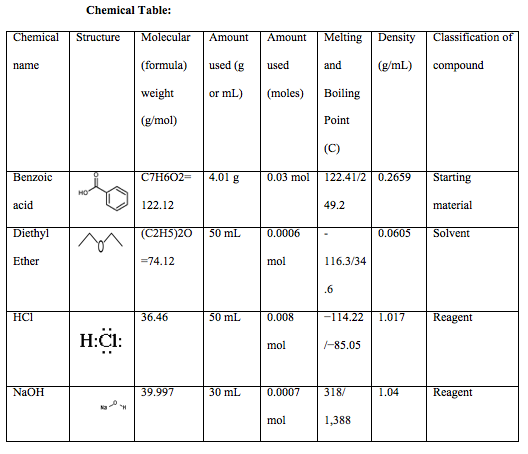
Extraction Of Benzoic Acid Odinity
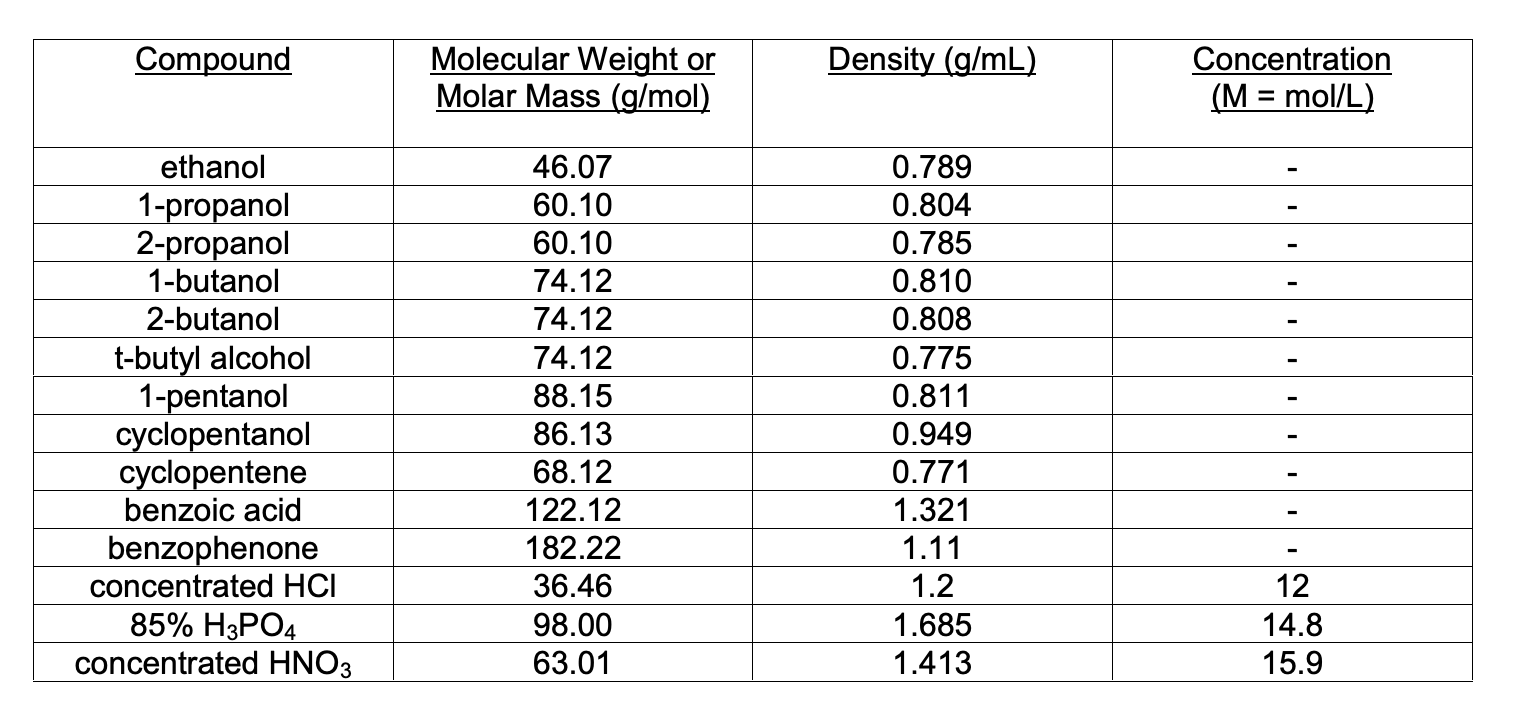
Solved Compound Molecular Weight Or Molar Mass G Mol Chegg Com
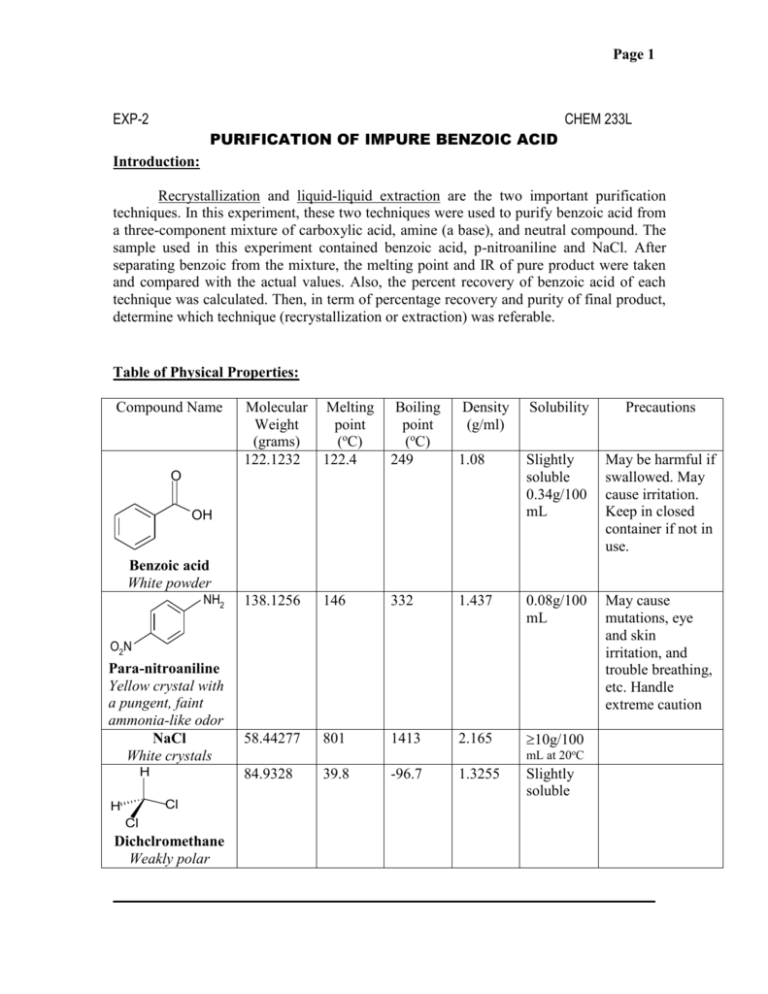
Purification Of Impure Benzoic Acid

Solved 2 23 A How Much Of The Primary Standard Benzoic Chegg Com

Benzoic Acid 65 85 0 C7h6o2 Density Melting Point Boiling Point Structural Formula Synthesis

How To Start Using Isopropyl Alcohol For Electronics Chemistry Chemical Science Medicinal Chemistry

Bond Repair Trial Kit Olaplex Sephora Hair Repair Olaplex Sephora

Solved Mass Of Benzoic Acid Used 5 040 Grams Volume Of Chegg Com

Oneclass How Many Moles Of Benzoic Acid Are Soluble In 145 0 Ml Of Waterat Room Temperature Mol Wt

Virtue Full Shampoo 240ml Heal Hair Damaged Hair Best Shampoos

Pin By Emily Anne On Receipts Lookin Like Phone Numbers In 2022 Leo Necklace Necklace Gold





Comments
Post a Comment